Am Fam Physician. 2017;96(5):293-299A
Author disclosure: No relevant financial affiliations.
Exercise stress testing is a validated diagnostic test for coronary artery disease in symptomatic patients, and is used in the evaluation of patients with known cardiac disease. Testing of asymptomatic patients is generally not indicated. It may be performed in select deconditioned adults before starting a vigorous exercise program, but no studies have compared outcomes from preexercise testing vs. encouraging light exercise with gradual increases in exertion. Preoperative exercise stress testing is helpful for risk stratification in patients undergoing vascular surgery or who have active cardiac symptoms before undergoing nonemergent noncardiac surgery. Exercise stress testing without imaging is the preferred initial choice for risk stratification in most women. Sensitivity and specificity increase with the use of adjunctive imaging such as echocardiography or myocardial perfusion imaging with single-photon emission computed tomography. Exercise stress testing is rarely an appropriate option to evaluate persons with known coronary artery disease who have no new symptoms less than two years after percutaneous intervention or less than five years after coronary artery bypass grafting. The Duke treadmill score has excellent prognostic value for exercise stress testing. Imaging is not necessary if patients are able to achieve more than 10 metabolic equivalents on exercise stress testing. Exercise stress testing is not indicated before noncardiac surgeries in patients who can achieve 4 metabolic equivalents without symptoms.
Exercise stress testing is used to detect inducible cardiac ischemia in symptomatic intermediate-risk patients who can exercise and who have interpretable electrocardiography results. 1 Risk is determined by American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines for stable ischemic heart disease or the Diamond and Forrester score to assess pretest probability of coronary artery disease (CAD; Table 1). 1 , 2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Preoperative exercise stress testing for risk stratification before noncardiac surgery is not indicated if the patient is able to achieve 4 or more metabolic equivalents without symptoms. | C | 1 , 5 |
| Exercise stress testing is helpful for risk stratification in patients undergoing vascular surgery and in those who have active cardiac symptoms before undergoing nonemergent noncardiac surgery. | C | 1 , 5 |
| Exercise stress testing is not recommended in asymptomatic patients to screen for coronary artery disease. | C | 3 , 6 , 7 |
| Adjunctive imaging increases cost without improving prognostic value in patients who can achieve more than 10 metabolic equivalents during exercise stress testing. | B | 19 , 21 |
| Recommendation | Sponsoring organization |
|---|---|
| Do not perform stress cardiac imaging or advanced noninvasive imaging in the initial evaluation of patients without cardiac symptoms unless high-risk markers are present. | American College of Cardiology |
| Do not perform cardiac imaging for patients who are at low risk. | American Society of Nuclear Cardiology |
| Avoid using stress echocardiography on asymptomatic patients who meet low-risk scoring criteria for coronary disease. | American Society of Echocardiography |
| Avoid cardiovascular stress testing for patients undergoing low-risk surgery. | Society for Vascular Medicine |
| Patients who have no cardiac history and good functional status do not require preoperative stress testing before noncardiac thoracic surgery. | Society of Thoracic Surgeons |
| Do not obtain baseline diagnostic cardiac testing or cardiac stress testing in asymptomatic stable patients with known cardiac disease (e.g., coronary artery disease, valvular disease) undergoing low- or moderate-risk noncardiac surgery. | American Society of Anesthesiologists |
| Do not perform routine annual stress testing after coronary artery revascularization. | Society of Nuclear Medicine and Molecular Imaging |
| Age (years) | Sex | Typical/definite angina pectoris | Atypical/probable angina pectoris | Nonanginal chest pain |
|---|---|---|---|---|
| ≤ 39 | Male | Intermediate | Intermediate | Low |
| Female | Intermediate | Very low | Very low | |
| 40 to 49 | Male | High | Intermediate | Intermediate |
| Female | Intermediate | Low | Very low | |
| 50 to 59 | Male | High | Intermediate | Intermediate |
| Female | Intermediate | Intermediate | Low | |
| ≥ 60 | Male | High | Intermediate | Intermediate |
| Female | High | Intermediate | Intermediate |
The standard Bruce protocol is preferred for exercise stress testing 3 (eTable A). Its outcomes are well validated, and exercise capacity measured in metabolic equivalents (METs) has good prognostic value. The Bruce protocol can be modified for patients with predicted poor exercise capacity by adding two warm-up stages before the first stage. The Naughton protocol allows for a more gradual increase in exertion and uses shorter stages, increasing the likelihood of diagnostic results in older and deconditioned patients. 3 This article reviews indications for and answers common questions about exercise stress testing.
| Protocol | Miles per hour | Grade (%) | Metabolic equivalents |
|---|---|---|---|
| Standard Bruce protocol (3 minutes per stage) | |||
| Stage 1 | 1.7 | 10 | 4 |
| Stage 2 | 2.5 | 12 | 7 |
| Stage 3 | 3.4 | 14 | 10 |
| Stage 4 | 4.2 | 16 | 13 |
| Stage 5 | 5.0 | 18 | 16 |
| Stage 6 | 5.5 | 20 | NA |
| Stage 7 | 6.0 | 22 | NA |
| Modified Bruce protocol (3 minutes per stage) | |||
| Stage 1 | 1.7 | 0 | 2 |
| Stage 2 | 1.7 | 5 | 3 |
| Stage 3 | 1.7 | 10 | 4 |
| Stage 4 | 2.5 | 12 | 7 |
| Stage 5 | 3.4 | 14 | 10 |
| Stage 6 | 4.2 | 16 | 13 |
| Stage 7 | 5.0 | 18 | 16 |
| Stage 8 | 5.5 | 20 | NA |
| Stage 9 | 6.0 | 22 | NA |
| Naughton protocol (2 minutes per stage) | |||
| Stage 1 | 1.0 | 0 | 1 |
| Stage 2 | 2.0 | 0 | 2 |
| Stage 3 | 2.0 | 3.5 | 3 |
| Stage 4 | 2.0 | 7 | 4 |
| Stage 5 | 2.0 | 10.5 | 5 |
| Stage 6 | 2.0 | 14 | 6 |
| Stage 7 | 2.0 | 17.5 | 7 |
Exercise stress testing is commonly used for the detection of CAD in patients with chest pain or dyspnea on exertion who are at intermediate risk of acute coronary syndrome. Exercise stress testing reduces costs of hospitalization without worsening outcomes in patients presenting to the emergency department with chest pain and negative cardiac enzymes. Additional indications for exercise stress testing include prediction of cardiovascular events, assessment of chronotropic competence, evaluation of exercise-induced symptoms, evaluation of unexplained syncope in patients at intermediate to high risk of CAD, and assessment of response after medical or surgical interventions in patients with valve disease, arrhythmias, or other heart diseases. Consensus opinion from the ACCF/AHA is that exercise stress testing can be used for exercise prescriptions, but data on patient-oriented outcomes are lacking.
The AHA states that early exercise stress testing in emergency departments and chest pain units is safe, accurate, and cost-effective because of fewer hospital admissions. 3 In a prospective cohort study of 3,552 patients in chest pain units who had low Diamond and Forrester scores, none had a positive stress test. 4 Another study evaluated intermediate-risk patients presenting to the emergency department who had no known CAD and in whom acute coronary syndrome was excluded with two negative cardiac enzyme tests performed six hours apart. 2 Exercise stress testing stratified intermediate-risk patients to a near zero short-term risk of acute coronary syndrome. A retrospective analysis of 3,987 patients younger than 40 years who were at intermediate risk of CAD and in whom myocardial infarction (MI) had been excluded found that exercise stress testing was of minimal value given the 0.4% incidence of positive findings. 5
Preoperative exercise stress testing is not indicated for risk stratification before non-cardiac surgery in patients who are able to achieve a minimum of 4 METs (e.g., walking up one flight of stairs) without cardiac symptoms, even if they have a history of CAD. 1 , 5 Exercise stress testing is helpful for risk stratification in patients undergoing vascular surgery and in those who have active cardiac symptoms before undergoing nonemergent noncardiac surgery. 1 , 5 Patients with poor functional capacity (unable to achieve 4 METs) should undergo stress echocardiography or exercise single-photon emission computed tomography (SPECT) before undergoing vascular surgery or a kidney or liver transplant. 1
Activities greater than 6 METs are associated with an increased risk of acute coronary syndrome. Experts recommend that deconditioned patients with diabetes mellitus, men older than 45 years or women older than 55 years, and those with two or more risk factors for CAD undergo exercise stress testing before starting a vigorous exercise program. However, no studies have compared outcomes from preexercise stress testing vs. encouraging light exercise with gradual increases in exertion. 3
Exercise stress testing is generally inappropriate for detection of ischemia in asymptomatic patients with no history of revascularization. Absolute contraindications include MI in the previous two days, ongoing unstable angina, uncontrolled cardiac arrhythmia with hemodynamic compromise, and symptomatic severe aortic stenosis (Table 2). 1 , 3 One in 10,000 exercise stress tests results in sudden cardiac death or hospitalization.
| Absolute contraindications |
| Active endocarditis |
| Acute aortic dissection |
| Acute myocarditis/pericarditis |
| Decompensated heart failure |
| Inability to exercise |
| Myocardial infarction in previous two days |
| Ongoing unstable angina |
| Symptomatic severe aortic stenosis |
| Uncontrolled cardiac arrhythmia with hemodynamic compromise |
| Relative contraindications |
| Acquired complete heart block (left bundle branch block) |
| Hypertrophic obstructive cardiomyopathy with severe resting gradient |
| Known left main coronary artery disease |
| Recent stroke or transient ischemic attack |
| Resting systolic blood pressure > 200 mm Hg or diastolic blood pressure > 110 mm Hg |
| Tachyarrhythmia with uncontrolled ventricular rate |
Testing asymptomatic patients without a history of revascularization is not recommended. 1 , 3 The U.S. Preventive Services Task Force recommends against testing low-risk patients and found insufficient evidence for those at intermediate and high risk. 6 The American Academy of Family Physicians supports this recommendation. 7 A randomized controlled trial of asymptomatic patients 50 to 75 years of age who had type 2 diabetes and no known CAD found that screening with adenosine-stress radionuclide myocardial perfusion imaging did not reduce nonfatal MIs or cardiac deaths over five years compared with no screening. 8 Testing patients with no new symptoms less than two years after percutaneous coronary intervention or less than five years after coronary artery bypass grafting is rarely appropriate. 1
Exercise stress testing is often better at excluding CAD than confirming it. Testing without imaging is the primary initial choice for risk stratification for most women and men. Imaging is best used when there is a baseline abnormality in resting electrocardiography that would make interpretation of results difficult, if the patient has symptoms at rest, if anatomic cardiac features require evaluation, or if it is likely that the test results would be nondiagnostic (e.g., in patients with poor exercise tolerance due to severe osteoarthritis) and that further testing would be required. Adjunctive imaging is required in patients taking digitalis because of the high false-positive rate of exercise stress testing alone in these patients.
Stress echocardiography and exercise SPECT are appropriate in symptomatic patients at intermediate or high risk of CAD and in those with difficult-to-interpret electrocardiography results. 1 Symptomatic patients with a history of percutaneous coronary intervention or coronary artery bypass grafting should undergo exercise SPECT, stress echocardiography, or coronary angiography as clinically indicated. 1
A 2012 systematic review of 34 prospective studies found that exercise stress testing and stress echocardiography were better at excluding CAD than confirming it (likelihood ratio [LR] of ruling out CAD via exercise stress testing = −0.34; 95% confidence interval [CI], 0.28 to 0.41; LR for stress echocardiography = −0.24; 95% CI, 0.17 to 0.32). 9 Of the two testing modalities, stress echocardiography was better at ruling in CAD (LR = 7.94 vs. 3.57 for exercise stress testing). 9 Sensitivity and specificity for CAD detection increase when imaging is performed with exercise stress testing (Table 3). 10 – 14 The prevalence of severe CAD is higher in older patients; exercise stress testing has a sensitivity of 84% in this population but a decreased specificity of 70%. 3 SPECT is no better at detecting severe CAD than exercise stress testing, but it stratifies more intermediate-risk patients as low risk. 15 SPECT is superior to echocardiography for attaining images diagnostic for CAD in obese patients and in those with chronic obstructive pulmonary disease. 16
| Test | Sensitivity (%) | Specificity (%) | Limitations | Advantages |
|---|---|---|---|---|
| Cardiac catheterization | 98 | 82 | Invasive, requires radiation | Preferred test, allows for detection and intervention |
| Exercise single-photon emission computed tomography | 85 | 85 | Cannot assess myocardium or valves, heart rhythm irregularities may affect results, soft tissue attenuation artifacts, requires radiation | Assesses myocardial perfusion and regional/global function at rest and during stress, good prognostic data and negative predictive value |
| Exercise stress testing | 68 | 77 | Requires normal baseline electrocardiography, not recommended for patients with history of percutaneous coronary intervention or coronary artery bypass grafting | Less expensive, limited equipment required, good prognostic data and negative predictive value |
| Stress echocardiography | 79 | 87 | Image quality affected by body habitus and dependent on operator, limited time for imaging postexercise | Assesses cardiac structure, global and segment function at rest and during stress, relatively inexpensive, does not require radiation, good prognostic data and negative predictive value |
A randomized controlled trial of symptomatic women at intermediate risk of CAD showed no difference in event-free survival over two years of follow-up between those undergoing exercise stress testing vs. exercise SPECT. 17 However, exercise stress testing costs less. Systematic reviews show that because the median prevalence of CAD in women is less than that in men, a positive result on exercise stress testing indicates a lower probability of CAD (69% vs. 89%); however, negative results in women have better negative predictive value. 9 Exercise stress testing without imaging is the preferred initial choice for risk stratification in women.
In a 2014 randomized controlled trial comparing exercise stress testing alone and exercise stress testing with myocardial perfusion imaging, 965 patients younger than 65 years who had no known CAD, normal resting electrocardiography, and symptoms of CAD underwent exercise stress testing for risk stratification. Per the provisional exercise stress testing protocol, if they achieved maximal predicted heart rate or greater than 10 METs of exercise with a clinically and electrically negative exercise stress test result, no imaging was performed. 18 All-cause mortality was similar between those who underwent imaging and those who did not. No cardiac deaths occurred in those who underwent exercise stress testing alone.
Persons who achieve greater than 10 METs on exercise stress testing have an excellent prognosis, with a low prevalence of significant ischemia or CAD mortality. Further imaging in these patients increases cost without increasing prognostic benefit.
Patients with Duke exercise treadmill scores greater than 7 have a five-year survival rate of 93% compared with 67% for those with scores less than −11. 19 METs are the only treadmill-associated variable significantly related to all-cause mortality. 20 Decreased exercise capacity is associated with increased risk of MI, unstable angina, and coronary revascularization. 21 A 1-MET increase in peak period treadmill workload was associated with an 18% reduction in cardiac events in patients older than 65 years and a 14% reduction in younger patients. 22 Achievement of more than 10 METs on exercise stress testing equates to a low risk of death, regardless of imaging results. 22 A prospective study of 7,236 patients without known dilated cardiomyopathy or moderate valvular disease who achieved more than 10 METs on stress echocardiography found less than 1% CAD mortality per person-year of follow-up, regardless of the presence of wall motion abnormalities on exertion. 23 Similarly, patients who achieved at least 10 METs on exercise SPECT had an annualized cardiac mortality rate of 0.1% and combined cardiac death and nonfatal MI rate of 0.4%. 20 This suggests that when at least 10 METs are achieved, further imaging increases cost without increasing prognostic benefit. 20 , 23
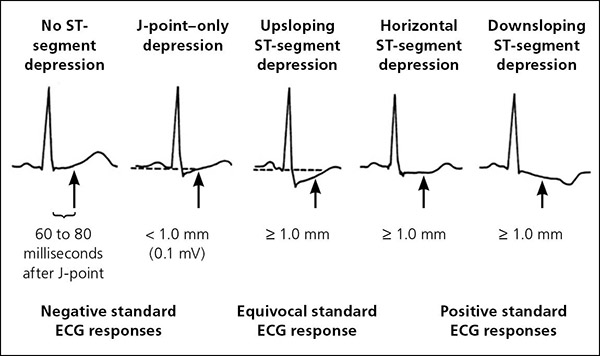
ST-segment elevation of more than 1 mm without preexisting Q waves is an absolute indication for termination of exercise stress testing, whereas a horizontal or downsloping depression of more than 2 mm measured 60 to 80 milliseconds after the J-point is a relative indication (Table 4). 3 Evidence of chronotropic incompetence by the inability of a patient's systolic blood pressure (BP) to rise above or drop below the resting systolic BP increases the risk of cardiovascular events. 24 A decrease in systolic BP of more than 10 mm Hg with other evidence of ischemia is an absolute indication to terminate testing. An isolated decrease in systolic BP is a relative indication.
| Absolute indications |
| Central nervous system symptoms (e.g., ataxia, dizziness, near syncope) |
| Decrease in systolic blood pressure greater than 10 mm Hg despite an increase in workload and accompanied by other evidence of ischemia |
| Moderate to severe angina |
| Signs of poor perfusion (e.g., cyanosis, pallor) ST-segment elevation (> 1.0 mm) in leads without preexisting Q waves because of prior myocardial infarction (other than aVR, aVL, and V1) |
| Sustained ventricular tachycardia or other arrhythmia (including second- or third-degree atrioventricular block) that interferes with normal maintenance of cardiac output during exercise |
| Technical difficulties in monitoring electrocardiography or systolic blood pressure |
| The patient asks to stop |
| Relative indications |
| Arrhythmias other than sustained ventricular tachycardia, including multifocal ectopy, ventricular triplets, supraventricular tachycardia, and bradyarrhythmias that have the potential to become more complex or to interfere with hemodynamic stability |
| Bundle branch block that cannot immediately be distinguished from ventricular tachycardia |
| Claudication, fatigue, leg cramps, shortness of breath, or wheezing |
| Decrease in systolic blood pressure greater than 10 mm Hg (persistently below baseline) despite an increase in workload and without other evidence of ischemia |
| Exaggerated hypertensive response (systolic blood pressure > 250 mm Hg or diastolic blood pressure > 115 mm Hg) |
| Heart rate > 85% of age-predicted maximum |
| Increasing chest pain |
| Marked ST-segment displacement (horizontal or downsloping> 2 mm, measured 60 to 80 milliseconds after the J-point) in a patient with suspected ischemia |
ST-segment elevation of more than 1 mm during stress identifies areas of ischemia in proximal coronary vasculature. 25 ST-segment depression of more than 2 mm does not localize anatomic ischemia, but when combined with clinical symptoms of ischemia suggests CAD (Figure 1). 3 The sooner ST-segment depression develops during testing and the longer it persists into recovery, the more severe the CAD. 26 As exercise increases cardiac output, systolic BP should increase. Inability to increase systolic BP suggests left ventricular systolic dysfunction or CAD. A prospective study (n = 44,000) of men and women, including blacks, with a mean age of 53 years showed a strong association between decreasing exercise systolic BP response, all-cause death, and MI. 27 The lower the patient's rise in systolic BP in response to exercise, the higher the incidence rate of MI per 1,000 person-years (increase of more than 20 mm Hg above baseline = 3.9 incidence rate [95% CI, 3.6 to 4.1], 1 to 20 mm Hg above baseline = 8.0 [95% CI, 7.0 to 9.1], and decrease from baseline = 12.5 [95% CI, 10.2 to 15.4]). 27 Therefore, it is recommended that exercise stress testing be discontinued if systolic BP decreases by more than 10 mm Hg.

Conversely, a hypertensive response to moderate-intensity exercise (systolic BP greater than 210 mm Hg in men or greater than 190 mm Hg in women) indicates a 1.36-fold greater rate of cardiovascular events and mortality (95% CI, 1.02 to 1.83; P = .039). 28 The AHA recommends termination of testing when systolic BP exceeds 250 mm Hg or when diastolic BP exceeds 115 mm Hg. 3 Reaching 85% of the maximal predicted heart rate (220 minus age) is a measure of adequate diagnostic exercise stress testing, but the AHA recommends that it not be used in isolation to terminate testing. 3 During exercise, the heart rate should increase by 10 beats per minute per 1 MET. Failure of the heart rate to increase and prolonged delay in returning to resting levels may affect prognosis and indicate CAD.
This article updates previous articles on this topic by Fletcher, et al., 29 and by Darrow. 30
The opinions and assertions contained herein are the personal views of the authors and are not to be construed as official or as reflecting the views of the U.S. Armed Services or their medical departments.
Data Sources: A PubMed search was completed using the MeSH function with the key phrase exercise stress test combined with at least one of the following terms: coronary artery disease detection or prognosis, stress echocardiogram, myocardial perfusion imaging, and SPECT. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were Essential Evidence Plus, the Cochrane Database of Systematic Reviews, and the websites of the U.S. Preventive Services Task Force and the American Heart Association. Search dates: April 2016 and May 2017.